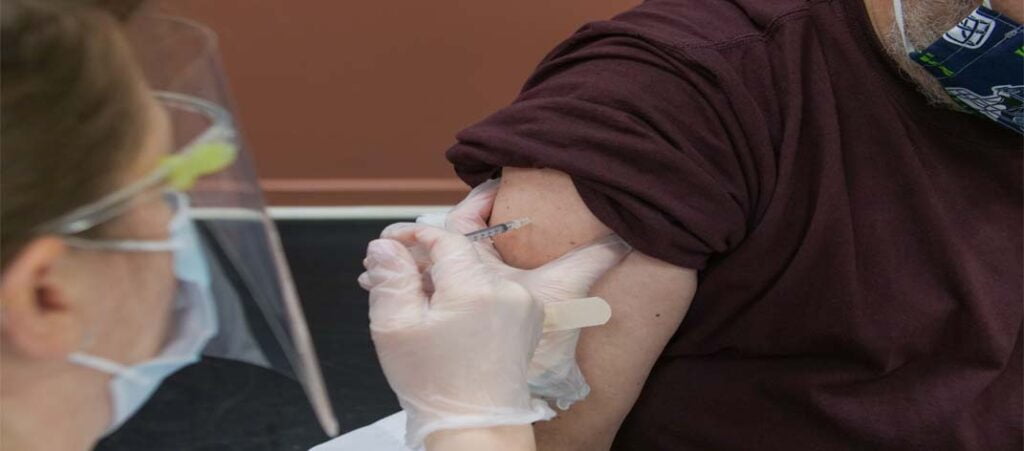
On October 3, 2023 the U.S regulators authorized another option for fall COVID-19 vaccination. The Food and Drug Administration has asked for an emergency usage of this vaccination. The health officials are hoping that enough people get the doses of the updated Novavax Shots ASAP. The updated Covid vaccine made by Novavax soon will be available for shipment to the offices, doctors, hospitals and pharmacies across the US. In a statement, Novavax said that this updated covid vaccine will be available in thousands of locations across the United States by next week.
The updated vaccines from Pfizer and Moderna which were made for adults and children aging 6 months and above began rolling out last month and now the Food and Drug Administration has added another option into their bucket. The updated Novavax shots are available for any one aging 12 years or above. Unlike mRNA vaccines made by Pfizer and Moderna, the Novavax shot includes protein and immune-boosting chemicals to protect people from Covid-19 and XBB.1.5 (A sub-variant of omicron).
Anyone can be vaccinated with this reformulated Novavax shot even if the person was previously vaccinated, said by the FDA in a statement. Also the person who was not vaccinated previously can also avail this vaccine. Further, the immunocompromised individuals can get additional doses of this vaccine as well. The dosage of the additional Novavax COVID-19 vaccine should be decided by the healthcare provider depending upon the individual’s clinical circumstances.
For the first time, the US Government is not buying the Covid vaccine shots; rather, the Novavax Shots are supposed to be free and covered under private insurance or Medicare. CDC has declared a temporary free Novavax Shots vaccination program for the uninsured or underinsured people. The US government is also encouraging vaccinations to combat the upcoming winter spike of Covid.
For more updates on latest health news please visit our website.